BNCT History
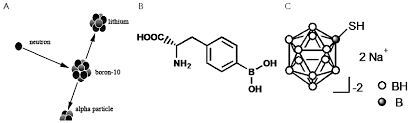
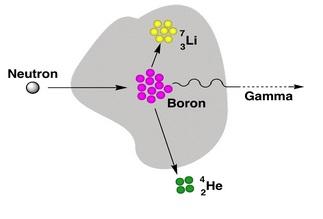
BNCT history may be traced back to 1935 (three years after Chadwick discovered neutrons in 1932) when Taylor and Goldhaber [2] described the reaction of thermal neutron capture by 10B boron nucleus. The resulting 11B nucleus decays to an alpha particle (4He) and a 7Li nuclei:
10B + nth →[11B] → α + 7Li + 2.31 MeV (1)
In 1936 Locher [3] proposed to use the above neutron capture reaction to cure cancer tumours. In 1951 Sweet [4] suggested to treat the most malignant brain tumours with BNCT. The first clinical trials were performed in Brookhaven National Laboratory (BNL) on polymorphic gliomas, soon afterwards some clinical tests were done also in Massachusetts Institute of Technology (MIT). Thermal neutrons of energies <0.4 eV were obtained from research reactors operated by the institutions. Non-selective Borax was used as the boron carrier. The tests were stopped in 1961 in view of unsatisfactory effects:
- neutrons rather weakly penetrated tissues
- boron accumulated not only in malignant cells, but also in blood
- some malignant cells managed to survive even after application of the highest doses permissible from the healthy tissue tolerance point of view
- scalp and brain blood vessels suffered damages.
A better boron carrier was needed.
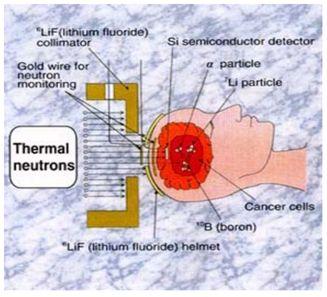
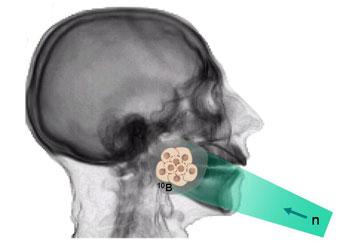
Next clinical tests were performed by Hatanaka [5] in Japan in 1968, who introduced BSH, a new boron compound of a better selectivity and ability to concentrate in malignant cells. Hatanaka increased also neutron penetration performing the BNCT procedure intra-operative i.e. on an open scalp (see Fig. 1). The effects were better, however, the obtained survival rate did not differ from that obtained using the conventional therapy. Thermal neutrons could not cure more deeply located tumours. BNCT was then applied to 120 patients.
In the next step neutron energy was increased to the epi-thermal range (0.4 eV – 10 keV). An increased neutron penetration translated into less damage to surface tissues and higher dose deposited into deeper-located tumours. Advancements in boron carrier technology resulted in development of BPA-f (Borophenylalanine-fructose), a molecule capable to penetrate cell membranes and that way to place boron atoms inside cells. New phase of clinical tests started in 1994 at BNL and MIT. Interest rose also in Europe: starting 1996 the BNCT treatment was tested in Petten (the Netherlands) and in some centres in Finland.
Just like in any other radiotherapy treatment, modern computers and advancements in medical imaging technologies (MRI, PET and the like) have significantly improved BNCT therapy planning. Current topics of research on BNCT include: neutron beam quality improvements, accurate neutron dosimetry, new boron carriers, alternative sources of epithermal neutrons (1 eV – 10 keV) of a deep penetration.
- Chadwick, J., (1932), Proc. Roy. Soc. Lond. A, 136, 692-708
- Taylor, H. J., Goldhaber M (1935), Nature (Lond.), 135, 341–348
- Locher, G. L., (1936), Amer. J. Roentgenol. Radium Ther., 36, 1–13
- Sweet, W. H. (1951), The uses of nuclear disintegration in the diagnosis and treatment of brain tumor, New England Journal of Medicine, 245 (23): 875–8, doi: 10.1056/NEJM19511206245230
- H. Hatanaka, (1986), Clinical experience of boron-neutron capture therapy for gliomas – a comparison with conventional chemo-immuno-radiotherapy, In: H. Hatanaka ed Boron Neutron Capture Therapy for Tumours. Nishimura Co., Niigata 349-379 http: //www. osaka-u. ac. jp/en/news/ResearchRelease/2013/09/20130906_1
- Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res 1999;151:1–18
- Morris GM, Coderre JA, Hopewell JW., et al. Response of the central nervous system to boron neutron capture irradiation: evaluation using rat spinal cord model. Radiother Oncol 1994;32:249–55
- Morris GM, Coderre JA, Hopewell JW., Micca PL, Rezvani M. Response of rat skin to boron neutron capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother Oncol 1994;32:144–53
BNCT physics
10B stable boron nuclei introduced to malignant cells are irradiated with low energy neutrons. Neutron energy is selected depending on the depth at which the to-be-destroyed cells are located. Thermal neutrons (energy < 0.05 eV) may be used close to the body external surface, more deeply penetrating epithermal neutrons (energy from 1 eV to 10,000 eV) – for more deeply located tumours. Neutrons traversing body tissues slow down. Once their energy drop down to the thermal region, capture-on-boron reaction cross section dramatically increases. Having captured a neutron, stable 10B nucleus transforms into meta-stable 11B nucleus, which almost instantly decays along one of the following paths:
10B + nth → 4 He2+ + 7Li 3+ + 2.79 MeV (6%)
10B + nth → 4 He2+ + 7Li 3+ + 2.31 MeV + γ (0.48 MeV) (94%)
Both particulate reaction products (7Li and α-particle) transfer their energy into the surrounding tissue at a high Linear Energy Transfer (LET) rate, so they are stopped already after 5 and 9 µm, respectively. Such small ranges are comparable with size of many malignant cells.
If boron atoms have been selectively introduced into malignant cells only, dose of high LET (hence lethal for cells) radiation will be localized within such cells only, surrounding healthy tissue will remain intact.
BNCT is a promising therapy in case of cancers resistant for traditional therapies, mainly glioblastomas and melanomas. Glioma is a very malignant brain cancer (patient survival time counted in months). Melanoma is a skin cancer known of frequent metastases to brain. Cancers of both these types are usually disseminated and oxygen-poor, which makes both surgery and chemotherapy difficult if not impractical. Chemotherapy of gliomas is also ineffective due to the blood-brain barrier. Selective deposition of lethal short-range radiation within malignant cells only may be an effective technique to treat such cancers. However, not all shortcomings of the BNCT technique have yet been overcome. The key issues are: (i) it is still difficult to generate and control beams of neutrons of therapeutic quality; (ii) boron carriers are still not ideally selective/adequately efficient.
Source: http://nanomed.missouri.edu/institute/research/BNCT.html
BNCT chemistry
Boron carriers must be low-toxic substances selectively acquired by malignant cells.
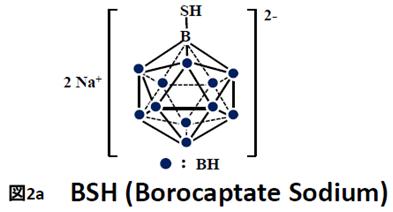
BSH (di-sodium undecahydro-mercapto-closo-dodecacarborate Na2B12H11SH) and BPA (p-boronphenylalanine C9H12BNO4) were the first Boron compounds used in BNCT (see Fig.2). Each of the compound uses another mechanism along which the B-containing molecules enter malignant cells.
BSH passively diffuses from blood stream into malignant cells in brain. The diffusion is possible because malignant cells disrupt the Blood Brain Barrier normally at work within the brain. Simultaneously BSH cannot penetrate healthy cells that do not disturb the barrier. Studies on that mechanism have shown that BSH can selectively penetrate brain cells if the cancer/blood ratio is within the 1:1 to 2:1.6 range [2].
BPA is a boron-labelled amino acid. Such bio-molecules are actively acquired by malignant cells that transport them through their membranes at rates much higher than the rates observed in healthy cells – just to sustain their extraordinarily large growth rates. Concentration of BPA in malignant cells is usually 2-4 times higher than in blood cells and in other healthy cells.
Unfortunately, neither BPA nor BSH penetrate malignant cells uniformly. Therefore distribution of boron – hence distribution of the deposited radiation doses – is not uniform throughout the cells. In consequence some malignant cells survive the therapy. Besides, selectivity is not perfect: some BPA or BSH penetrate also healthy cells. In consequence neutron dose must be limited. To overcome these shortcomings, some attempts have been made to simultaneously administer both BPA and BSH in the hope that the two different penetration mechanisms would supplement each other. Alas, the results were not satisfactory.
Better results were obtained with boron-loaded liposome and boron-loaded porphyrins.
Various liposomes are used as carriers not only in cancer therapy since they are bio-compatible with cell membranes and exhibit low toxicity. Normally blood vessel walls are weakly transparent for liposomes. However, if some inflammation appears within the organism (because of a bacterial/viral infection, or because of a neoplasmatic process), individual cells in blood vessel walls become separated by quite large gaps so that liposomes could leave the vessels and gather at the the inflammation place. All in all, boron-loaded liposomes can accumulate within cancer tumours. The mechanism is referred to as passive accumulation of liposomes.
Porphyrins are aromatic tetrapyrrole compounds. Some of them, for example chlorophyll and hem, naturally occur in plants and animals. They show significant affinity to neoplasmic tissue, low cytotoxicity, and can easily bind large amounts of boron. They are used as carriers also in Photodynamic Therapy.
1. Floberg J., The physics of boron neutron capture therapy: an emerging and innovative treatment for glioblastoma and melanoma, http://digitalcommons.carleton.edu/pacp/8
2. J.A. Coderre, J.C.Turcotte, K.J.Riley et al., Technology in Cancer Research & Treatment 2 (5), 355 (2003)
BNCT Radiobiology
To evaluate deposited dose is a pretty complicated task. Products of the 10B (n, α) 7Li reaction include photon, α-particle, and recoil nucleus. Other reactions of neutrons with healthy tissues include:
14N (n, p) 14C
1H (n, γ) 2D
14N + nth → 14C + 1H+ + 0.626 MeV
1H + nth → 2D + γ γ(γ2.2 MeV)
All the reaction products may interact both with malignant cells and with healthy cells. According to the common approach to dosimetry in BNCT, the following four components of the deposited dose are taken into account:
- protons from the 14N (n, p) 14C reaction (high LET radiation)
- protons from scattering of fast neutrons (high LET radiation)
- α-particles from the 10B (n, α) 7Li reaction and from decays of 4He nuclei (high LET radiation)
- γ-photons from the 1H (n, γ) 2D reaction and coming with neutron beam (low LET radiation).
The higher radiation LET, the larger biologic effect (in comparison with radiation of the same energy deposited along a longer path). That dependency is described by Relative Biological Effectiveness (RBE), a parameter of ionizing radiation defined as the following ratio: dose of the reference radiation producing the same biological effect to dose of the given radiation. BNCT treatment exposes both malignant and healthy cells to mixed radiation field of high-LET and low-LET components, even if beam of epithermal neutrons was ideal. Therefore the key merit of the therapy is its ability to selectively accumulate 10B in malignant cells to as much higher concentration than in neighbouring healthy cells as possible.
Compound Biological Effectiveness
Since biological effect depends on micro-distribution of 10B, more adequate indicator than RBE is needed in BNCT. Values of RBE coefficients determined for each component of the mixed radiation field (dependent on the used 10B carriers) have been named Compound Biological Effectiveness (CBE) factors. CBE values depend on numerous additional factors, such as the method/the way the boron carrier has been administered to the patient, distribution of boron in malignant/healthy tissue, or even size of the malignant cell nuclei.
![Struktura chemiczna BPA oraz BSH i CuTCPH oraz liposom z borem [Rolf F. Barth et al., Neurosurgery 44 (3), 433 (1999)]. Struktura chemiczna BPA oraz BSH i CuTCPH oraz liposom z borem [Rolf F. Barth et al., Neurosurgery 44 (3), 433 (1999)].](/sites/default/files/styles/large/public/projekt/chemia-bnct3.jpg?itok=T15-5S-I)